510(k) exemption for a 'kit'
hello
ASINs have been disabled for over 2 months now —B0CWQVVLDJ and B0CRLVD5QJ
other sellers are actively selling the exact same items I sell, individually, but don’t seem to be required to provide 510(k) documentation like I am. Their listings are still active, while mine are inactive.
The product I sell was titled ‘Portable Oxygen Tank Complete Set - Made in USA | Lightweight & Easy to Use,’which is now named 'E-Size Oxygen Tank Complete Set with Accessories - Made in USA | Lightweight & Easy to Use" which is essentially a ‘kit’—a combination of an oxygen tank and accessories. These accessories and different sizes of oxygen tanks are already being sold individually by other sellers on Amazon. I’ve designed and marketed this 'kit' or 'complete set' to make it easier for people to use an oxygen tank similarly in places like Mexico City, where oxygen tanks and their accessories are frequently used and purchased in bundled kits.
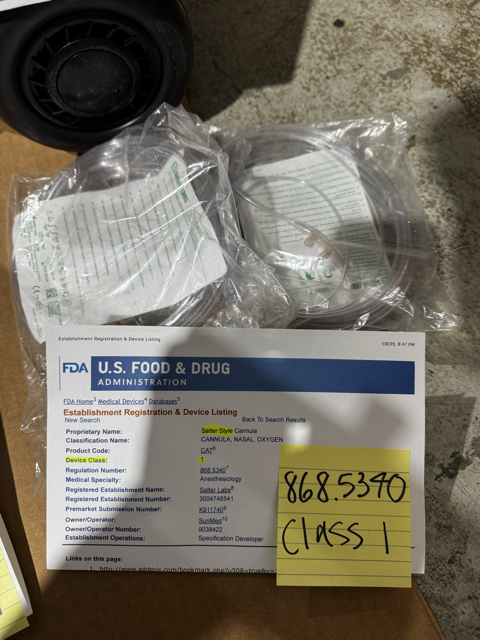
The "kit" or "complete set" I listed includes accessories that attach to an oxygen tank, making it portable and user-friendly. The components in the kit are classified as Class I under FDA guidelines and are 510(k) exempt based on their classifications. They’re not intended for professional use, meaning they can be sold OTC (Over-the-Counter) without needing 510(k) clearance, you can go and purchase all individual accessories and an oxygen tank at a local medical supply store for cash and don't need any license or documentation to purchase said items.
I understand that a policy might treat the whole kit as a professional-use-only device, but the individual pieces in my kit are each exempt on their own. They are currently sold separately online by different sellers, just under different brand names. So, the way I’ve packaged and marketed them shouldn’t require 510(k) documentation, since each piece is 510(k) exempt due to its Class I classification and 510(k) exempt status on fda.gov
On top of that, the business I work with is a registered FDA establishment—you can look it up on the FDA.gov website. I’ve tried explaining all this to an Amazon rep, but no luck. I’ve provided detailed information about all the products I sell and have images ready to show exactly what’s in the kit.
Essentially, if an Amazon rep looks at my listing, they’ll see it’s an oxygen tank with complete accessories all in a box. All of these individual items are actively being sold both FBM and FBA , just separately—not bundled together in a kit like mine.
Attached below are images of ASIN B0CRLVD5QJ.
ASIN B0CWQVVLDJ is a 416-liter tank, unlike the 682-liter tank shown below. Both tanks are proudly made in the USA by Catalina Cylinders, a leading supplier of American-made oxygen tanks. Additionally, instead of an oxygen tank carrying cart, listing B0CWQVVLDJ includes a cross-body size D bag, which I am approved to sell individually on my Seller Central account but I put currently out of stock. The same applies to the oxygen tank carrying cart shown in the images. I will also attach proof that other sellers are currently selling exactly what I sell and that my products do not require 510(k) documentation since they are 510(k) exempt but cannot do so since i can only upload 5 images at a time, would be happy to submit to an amazon rep that is handling my case directly if possible.




510(k) exemption for a 'kit'
hello
ASINs have been disabled for over 2 months now —B0CWQVVLDJ and B0CRLVD5QJ
other sellers are actively selling the exact same items I sell, individually, but don’t seem to be required to provide 510(k) documentation like I am. Their listings are still active, while mine are inactive.
The product I sell was titled ‘Portable Oxygen Tank Complete Set - Made in USA | Lightweight & Easy to Use,’which is now named 'E-Size Oxygen Tank Complete Set with Accessories - Made in USA | Lightweight & Easy to Use" which is essentially a ‘kit’—a combination of an oxygen tank and accessories. These accessories and different sizes of oxygen tanks are already being sold individually by other sellers on Amazon. I’ve designed and marketed this 'kit' or 'complete set' to make it easier for people to use an oxygen tank similarly in places like Mexico City, where oxygen tanks and their accessories are frequently used and purchased in bundled kits.
The "kit" or "complete set" I listed includes accessories that attach to an oxygen tank, making it portable and user-friendly. The components in the kit are classified as Class I under FDA guidelines and are 510(k) exempt based on their classifications. They’re not intended for professional use, meaning they can be sold OTC (Over-the-Counter) without needing 510(k) clearance, you can go and purchase all individual accessories and an oxygen tank at a local medical supply store for cash and don't need any license or documentation to purchase said items.
I understand that a policy might treat the whole kit as a professional-use-only device, but the individual pieces in my kit are each exempt on their own. They are currently sold separately online by different sellers, just under different brand names. So, the way I’ve packaged and marketed them shouldn’t require 510(k) documentation, since each piece is 510(k) exempt due to its Class I classification and 510(k) exempt status on fda.gov
On top of that, the business I work with is a registered FDA establishment—you can look it up on the FDA.gov website. I’ve tried explaining all this to an Amazon rep, but no luck. I’ve provided detailed information about all the products I sell and have images ready to show exactly what’s in the kit.
Essentially, if an Amazon rep looks at my listing, they’ll see it’s an oxygen tank with complete accessories all in a box. All of these individual items are actively being sold both FBM and FBA , just separately—not bundled together in a kit like mine.
Attached below are images of ASIN B0CRLVD5QJ.
ASIN B0CWQVVLDJ is a 416-liter tank, unlike the 682-liter tank shown below. Both tanks are proudly made in the USA by Catalina Cylinders, a leading supplier of American-made oxygen tanks. Additionally, instead of an oxygen tank carrying cart, listing B0CWQVVLDJ includes a cross-body size D bag, which I am approved to sell individually on my Seller Central account but I put currently out of stock. The same applies to the oxygen tank carrying cart shown in the images. I will also attach proof that other sellers are currently selling exactly what I sell and that my products do not require 510(k) documentation since they are 510(k) exempt but cannot do so since i can only upload 5 images at a time, would be happy to submit to an amazon rep that is handling my case directly if possible.





0 replies
CR_Amazon
@Seller_Gfj1xaFVVxNnz
I believe you created a duplicate discussion after you posted the first thread here and hereregarding the same topic.
Please only create one thread per question as it can create confusion and duplicate work for the Support Staff. Additionally creating multiple threads on the same topic could be seen as spam on the forums and go against forums guidelines.
I will be closing out this thread in order to avoid duplicative work. Any additional updates will be provided on the original post here.
CR_Amazon