Amazon misidentified our brand product as a medical device and requesting a 510K number (Case ID 14323873051)
Hello Mods
@Glenn_Amazon @Steve_Amazon @Atlas_Amazon @Sunnie_Amazon
I need some assistance as its been 30 days since our listing was removed and deleted from amazon. (Case ID: 14323873051)
Our product has been misclassified by Amazon as a “Spirometer”, which is a “ Professional-Use Only Medical Device”. Our product is a breathing exerciser similar to other exercise equipment, is meant for general training or muscle development. such as treadmills, free weights, etc… is not considered a medical device.
The device does not meet the definition under the regulations:
(a) Identification. An incentive spirometer is a device that indicates a patient's breathing volume or flow and that provides an incentive to the patient to improve his or her ventilation.
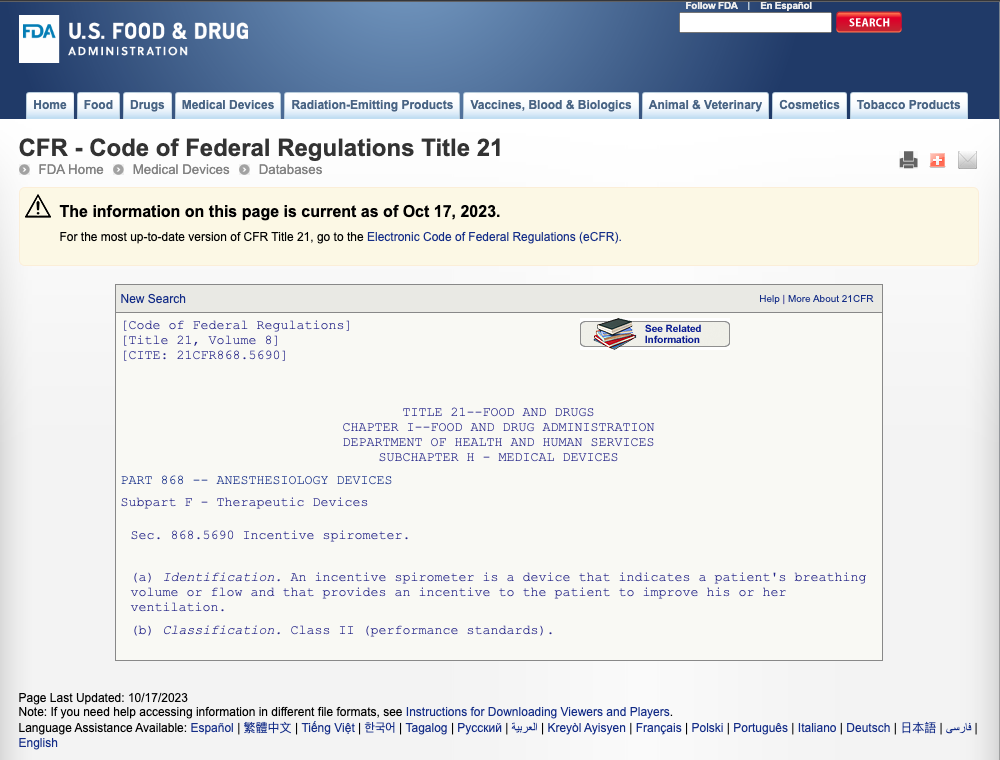
This item does not measure or check respiratory flow, volume or provide an incentive for customers to work on lung capacity. This item under its intended and stated use; is not regulated as a medical device under the FD&C regulations. And therefore, is exempted from any requirements of regulation, registration or device listing controls under the FDA.
This item is a 510k Exempt Device under regulation 890.5370
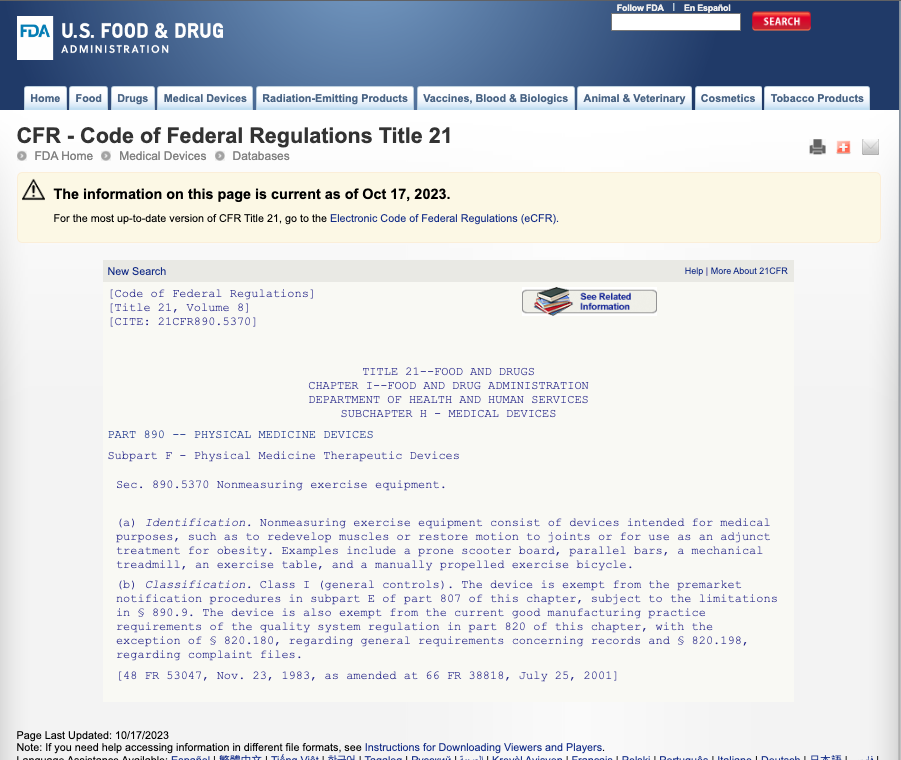
more information can be found here ..
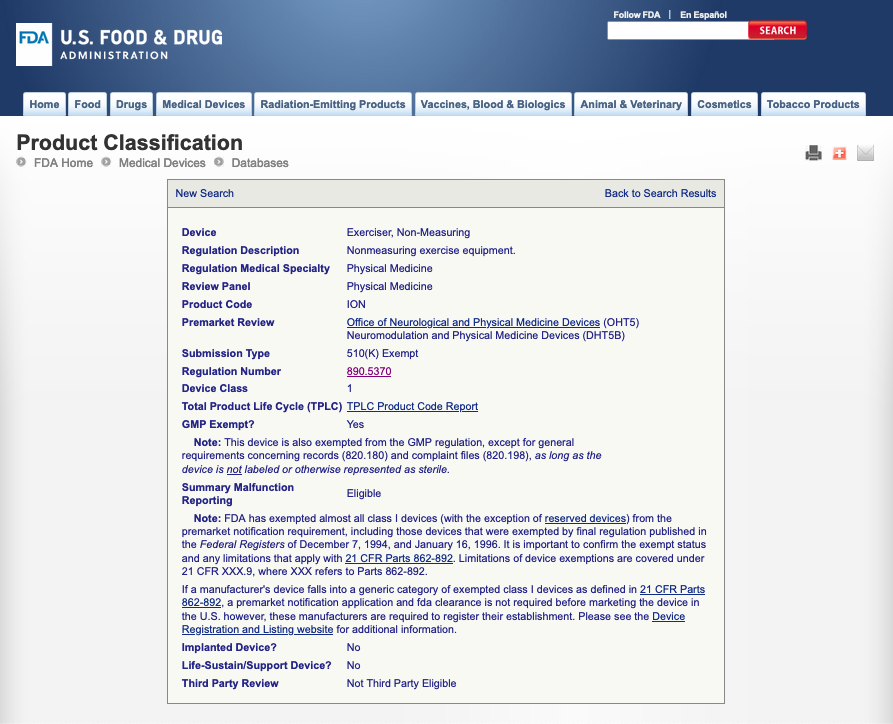
We have had our product reviewed by an accredited compliance expert, who has confirmed that the product is not a “medical device”, does not require registration with the FDA and does not require 510(k).
We believe this miscatagorization is due to a few keywords used on the product detail page and A+ content. We attempted to use a flat file to update the listing but were denied due to the listing being deleted/removed from amazon and we do not have access to it in any way. We believe In order for us to complete the appeal successfully, we need to remove a few inaccurate words used from the listing, but are unable to due to it being removed/deleted and we dont have any access to it. Its like a loop of death. We cant resolve this without being able to change some of the words used in the listing content.
The appeal keeps getting kicked back stating we need to supply a 510k number issued by the FDA. We believe this is an automated response and ask that a real person look at this case. because our product does not require a 510k number since it is exempt.
Amazon misidentified our brand product as a medical device and requesting a 510K number (Case ID 14323873051)
Hello Mods
@Glenn_Amazon @Steve_Amazon @Atlas_Amazon @Sunnie_Amazon
I need some assistance as its been 30 days since our listing was removed and deleted from amazon. (Case ID: 14323873051)
Our product has been misclassified by Amazon as a “Spirometer”, which is a “ Professional-Use Only Medical Device”. Our product is a breathing exerciser similar to other exercise equipment, is meant for general training or muscle development. such as treadmills, free weights, etc… is not considered a medical device.
The device does not meet the definition under the regulations:
(a) Identification. An incentive spirometer is a device that indicates a patient's breathing volume or flow and that provides an incentive to the patient to improve his or her ventilation.

This item does not measure or check respiratory flow, volume or provide an incentive for customers to work on lung capacity. This item under its intended and stated use; is not regulated as a medical device under the FD&C regulations. And therefore, is exempted from any requirements of regulation, registration or device listing controls under the FDA.
This item is a 510k Exempt Device under regulation 890.5370

more information can be found here ..

We have had our product reviewed by an accredited compliance expert, who has confirmed that the product is not a “medical device”, does not require registration with the FDA and does not require 510(k).
We believe this miscatagorization is due to a few keywords used on the product detail page and A+ content. We attempted to use a flat file to update the listing but were denied due to the listing being deleted/removed from amazon and we do not have access to it in any way. We believe In order for us to complete the appeal successfully, we need to remove a few inaccurate words used from the listing, but are unable to due to it being removed/deleted and we dont have any access to it. Its like a loop of death. We cant resolve this without being able to change some of the words used in the listing content.
The appeal keeps getting kicked back stating we need to supply a 510k number issued by the FDA. We believe this is an automated response and ask that a real person look at this case. because our product does not require a 510k number since it is exempt.
0 replies
Seller_rI7BZIczK8iAC
Why don't you just take a new UPC from GS1 and create a new listing? You used words in your listing that should make customers believe that this is a medical device.
There are people who say it IS possible to edit a removed listing. Personally I have never seen one who was successfull. You will just fight against windmills and loose precious time. Creating a new listing will avoid ALL these problems.
Seller_rI7BZIczK8iAC
Hundreds have tried to "remove forbidden words" from their deactivated listings. The problem is that you have no longer access to the listing and can't edit it.
Why are you against this simple solution? In two days you will have your listing alive. After one week you will see if the bots take it down again or not. The whole secret is in the wording.
Stevie_Amazon
Hi there @Seller_AoJZcus2Nz1yp,
Thank you for utilizing the Seller Forums and providing this insight into the situation you are facing with your ASIN.
While I can understand the intention of the product, there are also keywords or phrases which can impact a listing and cause them to be subject to the policy compliance, such as what has happened with your case in the item being flagged as a restricted product.
This particular policy states: "Medical devices are regulated by the Food and Drug Administration (FDA), which is the U.S. federal agency that is responsible for ensuring that medical devices intended for human use are safe and effective. A medical device is an instrument, apparatus, machine or related object used to diagnose, cure, treat, or prevent diseases in people or animals. Medical devices can also be used to change the structure or function of the body, such as stimulating hair growth." This means that any indication of an item claiming to be a treatment for, improve a body function, or cure something are going to fall under this particular policy, just as it will with the Food and Drug Administration.
One of the FDA criteria for a medical device is as follows: "...(B) intended for use in the diagnosis of disease or other conditions, or in the cure, mitigation, treatment, or prevention of disease, in man or other animals, or..."
Did this expert provide any official documentation to show this does not qualify as a medical device or is exempt from the 510(k) requirement?
I took a look into this case and was able to see the information you provide- including images of the packaging as well as an instruction manual. I see claims made on the product packaging and within the instruction manual that could be considered keywords for this specific policy.
The device does not meet the definition under the regulations:
(a) Identification. An incentive spirometer is a device that indicates a patient's breathing volume or flow and that provides an incentive to the patient to improve his or her ventilation.
With the claims I was able to see, the item might not directly be stating it is a spirometer. However, given the previous information from the Amazon policy and the FDA directly, the item is still meeting the criteria of being a medical device.
I was also able to see the listing within the case ID you had provided and see "Spirometry" is listed in the title.
"These products must comply with FDA’s Rx labeling requirements and they may only be sold to licensed healthcare practitioners. FDA recognizes that OTC and Rx classification can depend on a manufacturer’s intended use for the device. In these instances, the manufacturer is responsible for determining if the medical device should be sold to the general consumers (OTC) or if it can only be sold and operated by licensed healthcare practitioners (Rx)."
At this point, this item will not be allowed to be sold on Amazon.
Stevie.
Seller_AoJZcus2Nz1yp
Yes, we hired a expert that reviewed our product, we included their document stating that our product is 510k exempt and that its not a spirometer. They included all the FDA links showing that our product is a non measuring trainer which is 510k exempt
Seller_AoJZcus2Nz1yp
Stevie.. can you give some specifics.. what claims are you referring to?